THE FUTURE OF HEALTH MONITORING
Analysis: The potential of non-invasive health monitoring
Wearables such as the Oura or the Whoop are nowadays common accessories seen around the globe. There is a clear growing trend towards self-optimization and personal health monitoring. This is driving the rapid evolution of the technology behind wearable health-tracking tools. While these devices already generate vast amounts of data across a range of physiological parameters, including oxygen saturation, body temperature, heart rate, and more, they cannot yet provide insights into metabolism-specific biomarkers. So, while the current flow of information enables individuals to evaluate their lifestyle choices and enhance personal health monitoring, thereby facilitating proactive and predictive healthcare, it is crucial for the field to progress from relying on general indicators to assessing metabolism-specific biomarkers, such as glucose and lactic acid concentrations, in a scalable and non-invasive manner.
The Crucial Role of Biomarker Monitoring
Biomarkers serve as quantifiable indicators of biological processes, offering insights into individual well-being. Due to significant variance in biological characteristics across populations, assessing biomarkers on an individual basis yields more relevant insights than population averages. Examples of such biomarkers include sugars, lipids, proteins, acids, and ketones – each providing valuable data and insights into personal health.
Among these biomarkers, glucose holds particular relevance as the body’s main energy carrier. Its fluctuations are linked to a broad array of physiological processes, from how the body processes food to muscle mass and fat development, to energy production, and cognitive function. In the context of prediabetes and diabetes management, glucose monitoring becomes even more critical, serving as a tool for maintaining health, preventing complications, even saving lives.
Another essential biomarker is lactic acid, primarily produced in muscles and red blood cells. Lactic acid forms when the body metabolizes carbohydrates for energy under low oxygen conditions, such as during intense exercise, or in cases of infection or diseases that impair oxygen delivery to tissues. Lactic acid measurement is a standard method to determine the anaerobic threshold, the point at which oxygen supply meets demand at peak exercise intensity without reaching acidosis. Monitoring this threshold offers insights into fitness levels and physiological capacity, allowing individuals to optimize their endurance training and exercise intensity.
The Challenge of Current Invasive Methods
Currently, measuring these biomarkers typically requires invasive techniques, such as drawing blood or the use of (micro-) needles that remain embedded in the skin for extended periods. Although these methods provide the necessary sensitivity, specificity, and real-world reliability, they come with clear inconveniences and risks. As a result, mass adoption of continuous metabolic monitoring remains challenging. A true paradigm shift towards non-invasive biomarker measurement is essential to enabling the broad adoption of wearable health monitoring.
A Paradigm Shift: The Need for Non-Invasive Biomarker Measurement
Developing non-invasive wearable technologies for continuous metabolic biomarker monitoring faces several critical challenges. For mass adaption, any successful solution must meet four key requirements:
- Extreme Accuracy: Biomarkers must be detected and quantified with high precision – despite their low concentration and the complex environment of our bodies.
- Size: Devices must be miniaturized to a wearable format, which is an extreme miniaturization effort as most relevant technologies are starting from tabletop size.
- Practicality: The technology must be ready for everyday use and master a broad array of challenges: resilient enough to withstand temperature swings, sweat or hair, energy-efficient enough to operate all day, etc.
- Cost: To reach a large audience and penetrate the healthcare system, costs must be drastically reduced compared to existing lab-scale devices.
While some existing solutions meet some of these criteria, no technology has successfully achieved all four despite extensive research and significant investment. It was not for a lack of trying – numerous approaches explored included technologies like spectroscopy (across mid-infrared, near infrared, terahertz ranges), optical rotation, light scattering, radiofrequency, nuclear magnetic resonance, optical coherence tomography and many more…
Optical Spectroscopy: A Promising Path Forward
Among these explored techniques, optical methods stand out for their potential in non-invasive biomarker detection. Two approaches – infrared (IR) spectroscopy and Raman spectroscopy – leverage the interaction of light with biological materials to detect and measure specific biomarkers.
When light encounters a material, it can be reflected, scattered, or absorbed, depending on the light properties and the material’s characteristics. These interactions provide the foundation for measuring biomarkers non-invasively. In IR spectroscopy, biomarker detection relies on the absorption of infrared light, while Raman spectroscopy uses inelastic light scattering to identify biomolecular structures.
Raman spectroscopy: In essence, when light interacts with a molecule, it can transfer energy between the photon and the molecule, altering the rotational and vibrational energy of the molecule. This energy exchange creates shifts in the scattered light’s frequency, a decrease (Stokes-Raman scattering) or increase (Anti-Stokes Raman scattering) in frequency depending on whether the molecule absorbs or releases energy. The frequency shift depends on the vibrational modes of the chemical structure of the molecule, making Raman spectroscopy a label-free method for biomarker detection.
However, the Raman scattering cross-section decreases with longer wavelengths, reducing signal strength, although these wavelengths penetrate tissue more effectively and minimize autofluorescence – as fewer tissue components are excitable at these wavelengths, thereby improving sensitivity. Experimentally, monochromatic light illuminates the molecule, and scattered light is captured and analyzed by a spectrometer, producing a Raman spectrum unique to each molecule’s vibrations, enabling molecule identification.
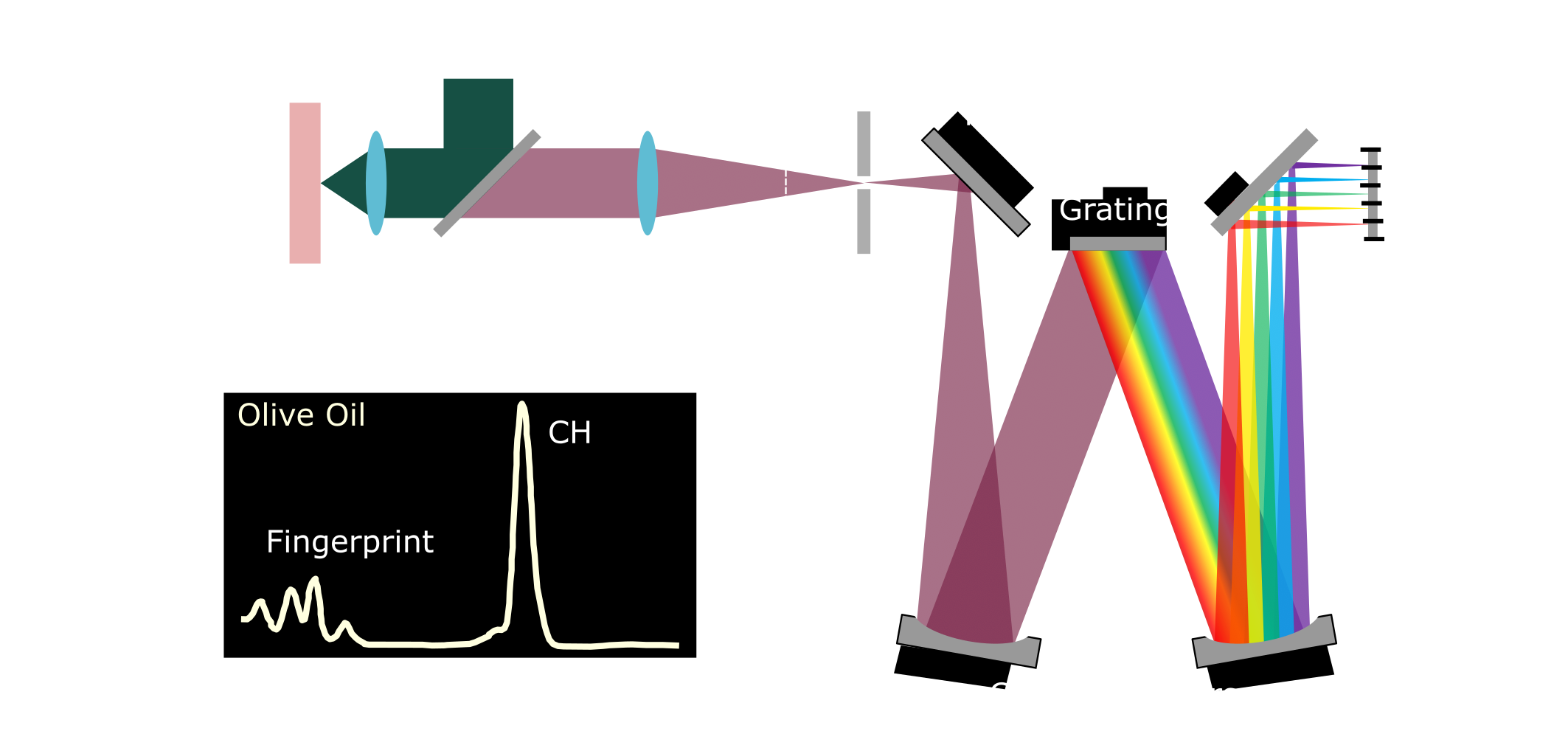
Figure 1: A) Schematic of a Raman spectroscopy setup. A monochromatic light source illuminates the sample, resulting in scattered light that is collected and directed to the spectrometer. Within the spectrometer, a grating disperses the light by wavelength, and a CCD detector captures the resulting spectrum. B) Example Raman spectrum of olive oil, adapted from [1], showing the Raman shift relative to the excitation wavelength. The spectrum highlights the fingerprint region and the characteristic peak corresponding to the CH stretch.
Mid-infrared spectroscopy (MIR): In MIR, mid-infrared light (2–20 µm) excites molecular vibrations, leading to photon absorption by specific biochemical bonds, reducing the detected beam’s intensity. Comparing the altered intensity to a reference allows for drawing a transmission spectrum specific to certain molecules. MIR requires a tunable mid-infrared source, often a quantum cascade laser, and long-wavelength-sensitive detectors like MCT-detectors. While effective, the setup remains bulky and costly, confining its use to lab environments.
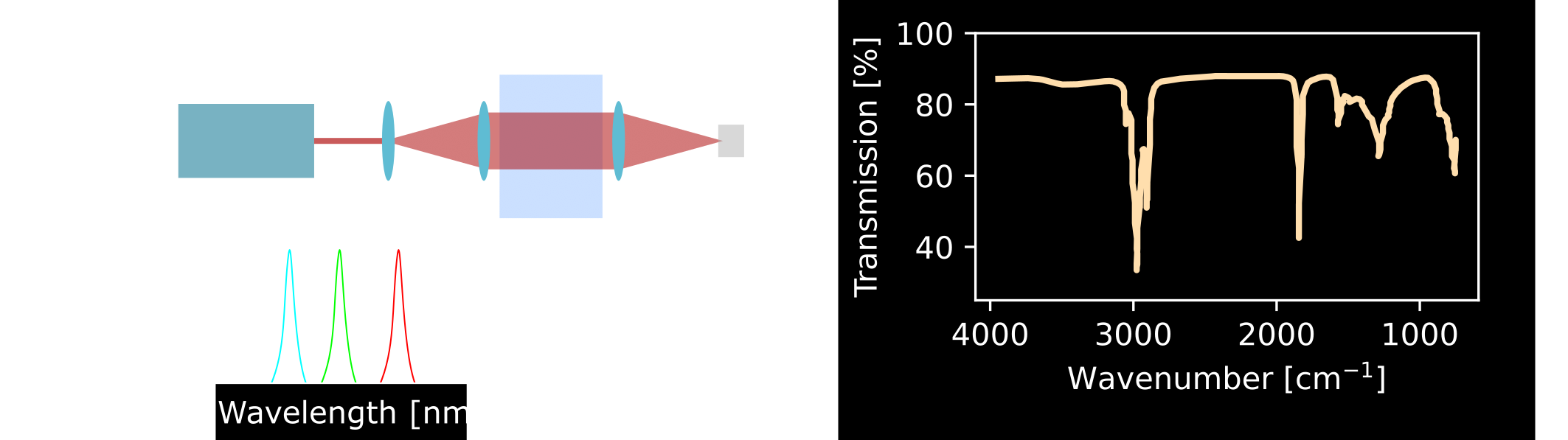
Figure 2: (A) Schematic of a basic mid-IR spectroscopy setup. A tunable light source illuminates the sample, and a detector measures the transmitted light. Comparing the transmitted light with that of a reference sample allows identification of absorbed wavelengths, which correspond to specific molecular bonds. (B) Example transmission spectrum of sunflower oil [2].
Simultaneously, while optical methods show potential, they too face challenges in delivering the required sensitivity and reliability across diverse real-world conditions.
A Dynamic Landscape of Opportunities
The field of non-invasive biomarker monitoring is dynamic, with various companies and academic researchers pushing the boundaries of what is possible. While current solutions have yet to meet all the rigorous standards required for everyday use, the rapid pace of innovation suggests that significant breakthroughs are on the horizon. The convergence of technological readiness and market demand makes this an exciting time for personalized healthcare.
Stay tuned as we move closer to scalable, user-friendly, wearable health monitoring technologies, poised to transform how we approach personal health and wellness!
—-
[1] B. Arús, et al. (2024). Macroscopic label-free biomedical imaging with shortwave infrared Raman scattering. https://doi.org/10.1101/2024.06.10.597863
[2] G. Shimamoto, et al. (2015). Simple Methods via Mid-IR or 1 H NMR Spectroscopy for the Determination of the Iodine Value of Vegetable Oils. https://doi.org/10.1101/2024.06.10.59786310.5935/0103-5053.20150111